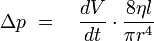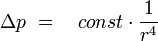Background knowledge
Scale and DSL
By using the Differential Scale Loop (DSL) the scale behaviour of mineral deposits can be studied. Two ionic solutions (brines) are mixed and pumped through a test capillary. By exceeding the solubility of at least one salt precipitates and crystals grow on the capillary's wall. Thus the diameter of the capillary decreases. Due to the chosen test conditions a laminar flow is caused inside the capillary.
For laminar flows applies Hagen-Poiseuille's law:
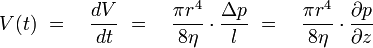
This shows that changes in pressure are depending of the forth power of the capillary radius, even very small changes in diameter cause measurable pressure changes. These changes in pressure are measured with the DSL and by this an indication for the beginning of scale formation is given.
The measuring principle provides that a tested scale inhibitor (SI) is injected with one of the solutions at a high concentration in the beginning. The concentration of SI is reduced in steps until a pressure rise is determined. The concentration of SI at the point of pressure rise is too low to be effective against scale formation. The step before this point is the lowest effective SI-concentration to avoid scale.
By measuring under a wide range of conditions regarding to temperature, pressure and volume flow rate, qualitative and quantitative comparisons of different inhibitors are possible as well as optimization of conditons for one special inhibitor.
Scale
In oil production the term scale covers not only the precipitations of calcium and magnesium carbonate but all occuring mineral deposits. Normaly this includes the carbonates and sulfates of the alkaline earth metalls calcium, strontium and barium. But also exotic deposits like metal carbonates (e.g. FeCO3), sulfides (e.g. FeS, ZnS, PbS), silicates or phosphates (Ca3(PO4)2) can occur.
Some criterias must be met before it comes to deposits:
The solubility product of salts must be exceeded by changes of the local conditions like temperature, pressure, pH-value or water composition.
Seed crystals must be existant otherwise the solutions stays in supersaturated condition and no or only amorphic precipitates can occur.
Crystal growth must be possible, kinetic inhibition must be small or missing.
Adhesion between crystals and wall must be possible so that the cystals can grow on the vessel wall (e.g. pipeline wall).
If at least one of these conditions does not occur the formation of scale is delayed or even avoided. For every condition different approaches are given for industrial oil production. Ions can be filtered out and pressure, temperature and pH-value can be varied in some degree. Also scale inhibitors are used to interfere with the formation of scales, e.g. masking ions with chelators or using chemicals which interfere in the crystallisation process. Different solutions are possible depending at on-site conditions.
Existing deposits can be removed in most cases. The chemical scale composition is crucial for the chosen condition. Normally the dissolution is forced by significant changes of pH-value or by added special chemicals.
For the frequently occuring scales of CaCO3 and BaSO4 see keywords calcium carbonate / barium sulfate.
Generally the avoidance of scale is preferred for economical reasons because it can be done during operation (continuous or discontinuous addition of chemicals). This way the running production does not to be interrupted.
Solubility
The Solubility is referring to the ability of a substance (solute) to dissolve in a specific solvent. The resulting alloy is called solution and is single-phase and homogeneous.
The quantitative Solubility can be expressed in different units. Normaly used are weight (g, kg), volume (mL, L) or amount of moles (mol) of dissolved substance in relation to weight (g/kg) or volume of solvent.
The qualitative Solubility is “ for thermodynamical reasons “ given for every substance in every solvent. In practice classifications are made that divides substances e.g. into poorly soluble, soluble and good soluble. These classifications are depending on the referring solvent. How good a substance is soluble depends on its and the solvents molecular characteristics.
Limit of solubility
Many substances have an upper limit of solubility. Up to this limit the concentration increases with the added amount of solute. Reaching the limit, the concentration stays at a constant level even if more of solute is added. The solution is saturated and a second phase (precipitation of added solids) occurs. Between the phases a solubility equilibirum is formed. The upper limit of solubility is temperatur-dependent (for gases also pressure-dependent), therefore given solubilities are valid only for standard conditions (p = 1013 hPa, t = 20 °C or 25 °C) if not otherwise identified.
Solubility product
The solubility product Ksp of a pure, dissolving substance in pure solvent is a constant at a given temperature. At equilibrium it is the product of the concentrations of the dissolving parts in the power of their stoichiometric coefficients.
Example for a totally dissolving salt AxBy:
KL(AxBy) = cx(A) * cy(B) [molx+y/Lx+y]
Supersaturation
A supersaturated solution is given if the equilibrium solubility is exceeded but the formation of a second phase is kinetically inhibited e.g. by missing activation energy or insufficient diffusion processes. This conditions can be commonly achieved by cooling of a saturated solution. Even though the supersaturation is metastable the solution stays in its single-phase condition as long as the kinetic inhibitation exceeds the thermodynamical term. If energy or e.g. seed crystals are added to the system, the equilibirum is disturbed and the thermodynamically favorable equilibirum can be obtained by a phase separation.
Seed crystals
Seed crystals are colloid solid particles in a fluid phase or disturbances at the phase interface (e.g. vessel wall). They ease the separation of a new phase out of a supersaturated solution. The nature of the phase separation (solid-liquid in case of crystallization or liquid-liquid) is not essential.
For the formation of a new phase the Gibbs free energy is a combination of the two terms for the thermodynamical controlled and the kinetical controlled work. For very small crystals the kinetical term is overweighed, so the crystal growth is kinetically inhibited or prevented. If seed crystals are added to a supersaturated solution the kinetical inhibition is bypassed, the thermodynamical term is overweighing. In consequence the crystals can grow.
Calcium carbonate
Molecular formula: CaCO3
Decomposition at 825 °C, CaCO3 → CaO + CO2
Poorly soluble in water , KL = 5 * 10-9 mol2/L2 (25 °C)
Soluble in diluted acids
If calcium carbonate precipitates out of a supersaturated solution it forms at first an amorphous deposit which transfers by-and-by to the crystalline form of calcite or at temperatures >30 °C of aragonite.
Avoiding of scale: by adjusting the temperature the solubility product can be influenced but pressure and pH-value offer a wider scope by interfering in the bicarbonate equilibirum. Alternatively calcium ions can be masked by chelatation.
Scale removal: The mentioned crystal forms of calcium carbonate are easy soluble in acids like diluted hydrochloric acid, acetic acid or citric acid:
CaCO3 + H3O+ → Ca2+ + HCO3-.
Barium sulfate
Molecular formular: BaSO4
Decomposition at 1580 °C, BaSO4 → BaO + SO2 + ½ O2
Poorly soluble in water, KL = 1,08 * 10-10 mol2/L2 (25 °C)
Barium sulfate is resistant to nearly all bases and mineral acids but is soluble in concentrated sulfuric acid. Scale occurs due to the very little solubility product nearly immediately after mixture of barium- and sulfate-containing brines.
Avoiding of scale: by adjusting the temperature the solubility product can be slightly influenced. By using scale-inhibitors barium and/or sulfate ions can be masked or crystallization can be inhibited / delayed.
Scale removal: changes in pH-value have no effect on the solubility of barium sulfate, the solubility in concentrated sulfuric acid is not an option under field conditions. Hence, chelators (like EDTA, DTPA, HEIDA) are used to dissolve precipitations. The barium ion forms a chelate complex with the chelator. These complexes are soluble in water. The chelation in comparison to the acid dissolution of carbonates is generally the slower reaction.
Carbonate-hydrogencarbonate (Bicarbonate) equilibrium
CaCO3 + H2O + CO2 → Ca(HCO3)2
In carbonate acid containing water an equilibrium is given between the poorly soluble calcium carbonate and the soluble hydrogencarbonate. By altering the amount of dissolved carbon dioxide the state of equilibrium can be moved.
If heated the solubility of CO2 in water decreases . The state of equilibrium moves to the left side of the chemical equation, calcium carbonate can precipitate (Le Chatelier's principle). The precipitating calcium carbonate crystallizes at seed crystals on the walls so that after longer time of crystallisation or numerous repetitions a deposit occurs. These crystals of calcium and/or magnesium carbonate are commonly called (lime)scale.